stimOS Product Portfolio
stimOS offers its products and services to small, medium-sized but also to larger enterprises.
Mimicking Bone Technology
Inspired by nature.
Developed by StimOS.
MBT is one technology concept addressing the most important needs of surgeons and patients demanding for high performance implant materials to be used in spinal, dental, CMF and other orthopedic applications.
01 MBT OSSEO
02 MBT BIOCIDE
03 MBT PROTECT
04 MBT ACTIVE
05 MBT DENTAL
MBT OSSEO is our „master“ surface functionalization technology. stimOS developed a covalently bonded 3D continuous biomimetic surface layer, specifically designed for inert implant materials. This development exhibits excellent combinations of surface free energy and mechanical stability. The combined implant and surface functionalization (MBT) shows high cyto-tolerance for bone constitution and ingrowth of bone cells. MBT is (a) bio-compatible, (b) avoids infections, (c) preserves healthy bone, (d) stimulates new bone formation, and results in an (e) overall high BIC (bone-to-implant contact), superior to all currently known surface materials or coating technologies.
01
PICTURE RIGHT:
Healthy bone surrounds the freshly placed implant in-vivo only two weeks after the operation.
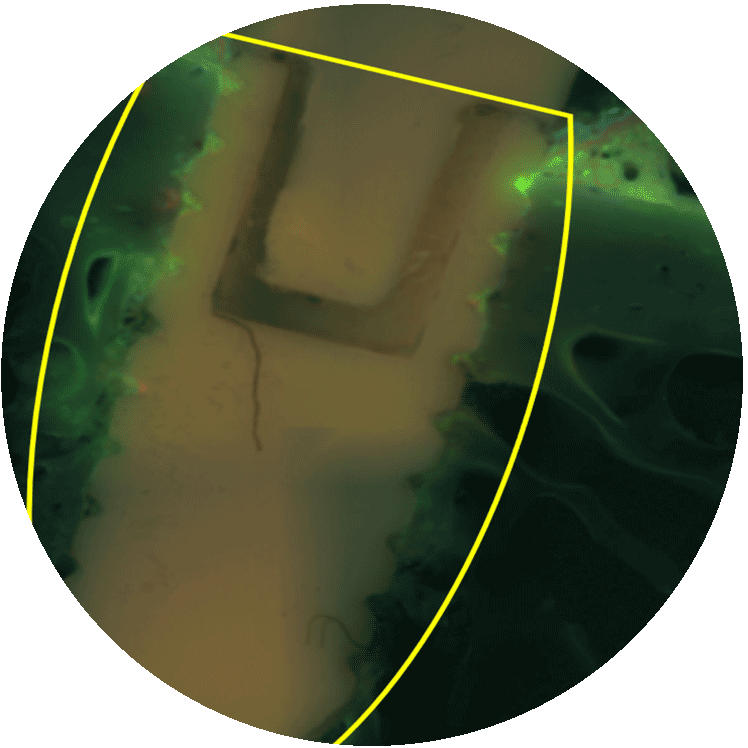

MBT BIOCIDE is our antibacterial variant combining both, superior osseointegrative characteristics with antibacterial properties. MBT biocide must be the surgeon‘s preventive choice if a risk for infection is already known for certain applications. MBT biocide is based on the biomimetic principles of osseointegration, combined with antibacterial properties, inspired by nature. MBT biocide does not incorporate any pharmaceutical components.
02
PICTURE LEFT:
In vitro cell tests proved that after 12 hours a dense cell layer occupies the implant, and collagen fibrils are formed.
MBT PROTECT is our anti-corrosion variant, combining both, superior osseointegrative features with protection against corrosion. In the case of implants and prosthetics, wear debris and ion release occurring due to the loss of material caused by bio-tribo corrosion of implant surfaces result in tissue inflammatory reactions. MBT protect avoids this corrosion and enhances the implant’s osseointegrative characteristics using one bio- inspired surface modification.
03
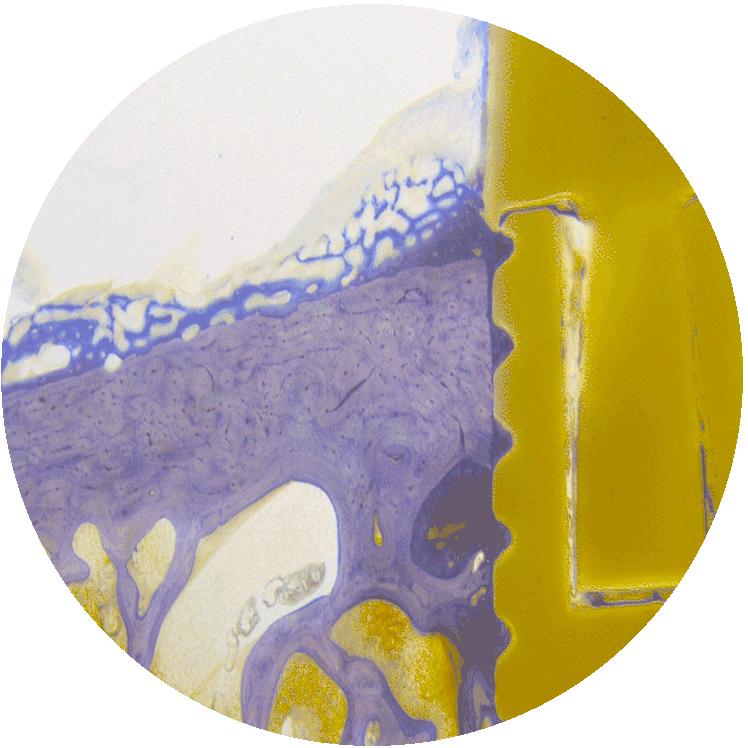
PICTURE RIGHT:
All MBT surface treatments result in a dense BIC that surrounds and firmly anchors the entire implant.
MBT ACTIVE is the variant of MBT surface functionalization, that offers the possibility of introducing active ingredients and drugs into the patient‘s body together with the implant and directly to the place where it is needed. As a patient-specific surface functionalization, drug delivery and its release can be controlled by MBT active. As a borderline product, the implant must be specifically evaluated depending on which function is the primary one: The mechanical functionality of the implant or the pharmaceutical characteristics of the active ingredient. Talk to us: Together, we will define the intended use and the approval strategy for your customized product.
04
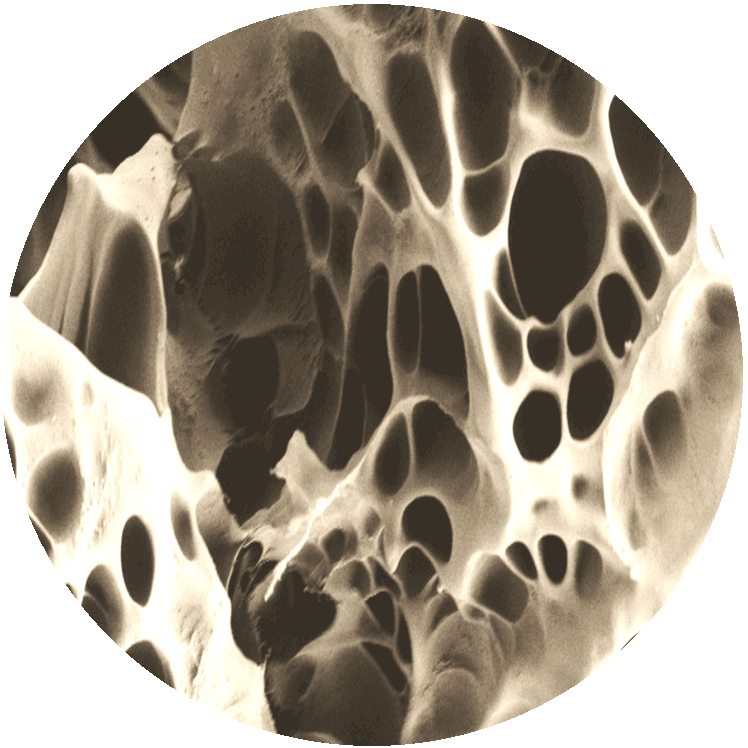
PICTURE LEFT:
MBT surfaces always build porous structures that guarantee best anchoring, conditions and nutritions for bone cells growing on the implant’s surface.
MBT DENTAL is the surface functionalization technology for dental applications. Based on hyaluronic acid it provides optimal conditions for implants placed in the jaw. MBT dental and all other variants of MBT technology family – that are available today – have been tested in cooperation with Charité Berlin and the University of Zurich. Testing was carried out in a comparative in-vitro and in-vivo setting comparing the surfaces of Titanium, HA-enhanced PEEK, PEEK and MBT technology. Both in-vivo and in-vitro MBT proved its superiority compared to currently available benchmark technologies.
05
PICTURE RIGHT:
MBT follows every implant’s surface using an engineered topography in the nanometer to micrometer range.

MBT – Proof of concept:
Animal Model Results
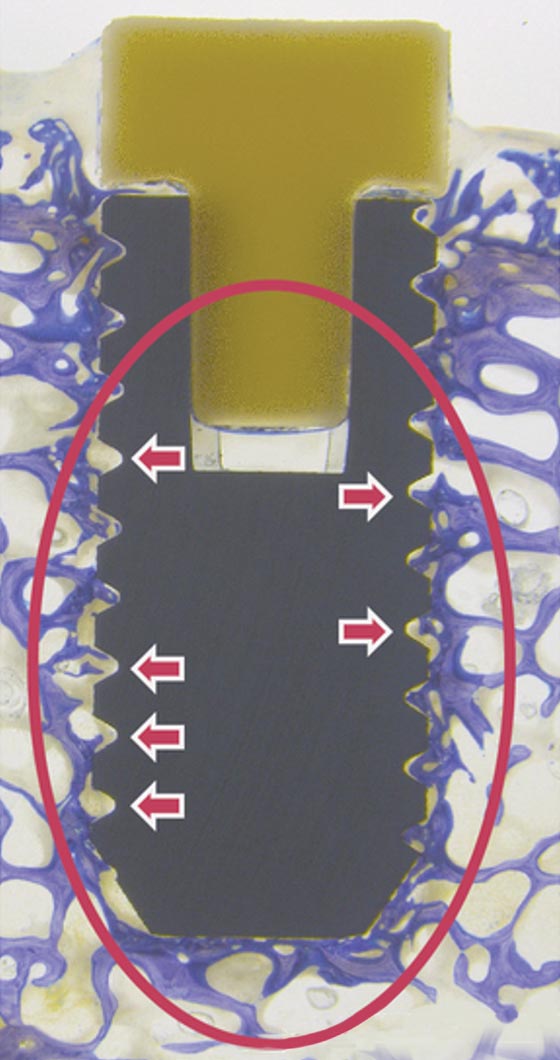
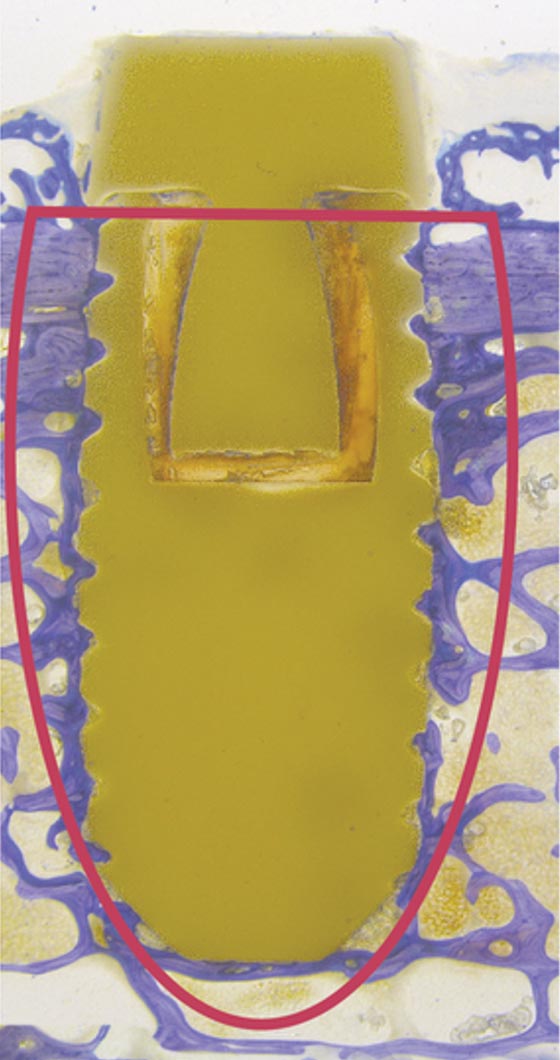
Titan
Golden Standard
Dense new bone is only oberstes in the upper part of the implant.
Screw thread is not filled homogenous with bone.
No stable anchorage of implant in the surrounding bone.
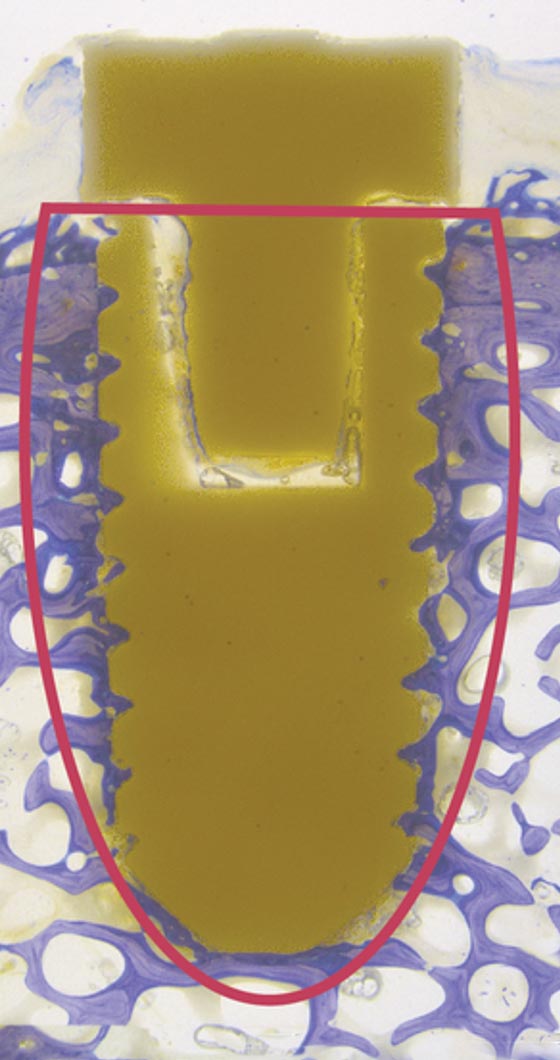
HA enhanced PEEK
Reference/Invibio
Bone is only oberstes on one side of the implant: Screw thread is not filled homogenous with bone.
No stable anchorage of implant in the surrounding bone.
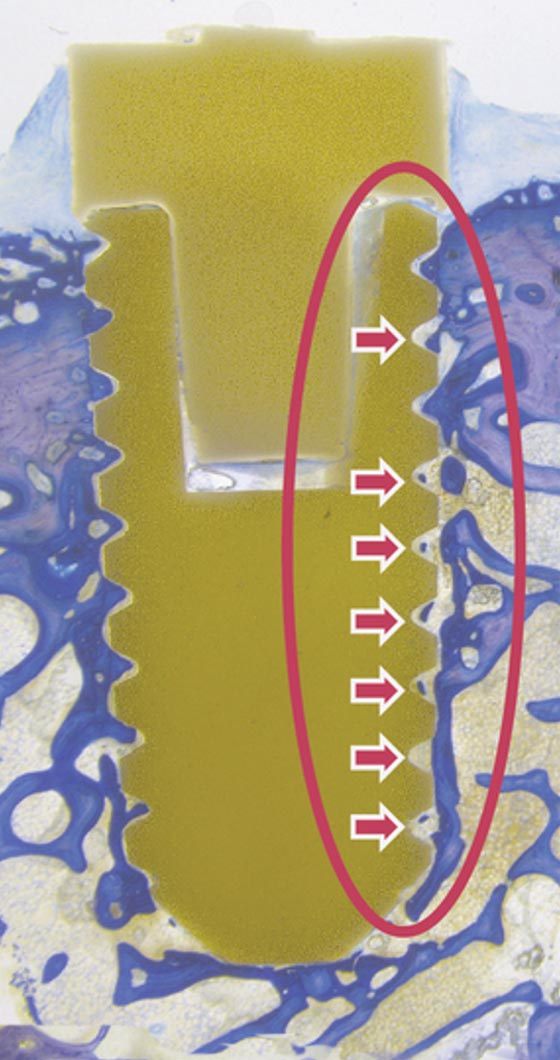
MBTv
Test-Device stimOS
Test implant is anchored completely in surrounding (new)bone.
Screw thread is fully filled: No fibrotic layer can be observed.
MBTg
Test-Device stimOS
Test implant is anchored stable in surrounding (new) bone.
Screen thread is fully filled: No fibrotic layer can be observed.
MBT – Proof of concept:
Animal Model Results
01
TITAN GOLDEN STANDARD:
Dense new bone is only oberstes in the upper part of the implant.
Screw thread is not filled homogenous with bone.
No stable anchorage of implant in the surrounding bone.

02
HA ENHANCED PEEK REFERENCE/INVIBIO:
Bone is only oberstes on one side of the implant: Screw thread is not filled homogenous with bone.
No stable anchorage of implant in the surrounding bone.

03
MBTv TEST DEVICE STIMOS:
Test implant is anchored completely in surrounding (new) bone. Screw thread is fully filled: No fibrotic layer can be observed.

04
MBTg TEST DEVICE STIMOS:
Test implant is anchored completely in surrounding (new) bone. Screw thread is fully filled: No fibrotic layer can be observed.

YOU NEED
NO COATING.
YOU NEED MBT.
spineFuse Implant System
Stealth Technology for Implants
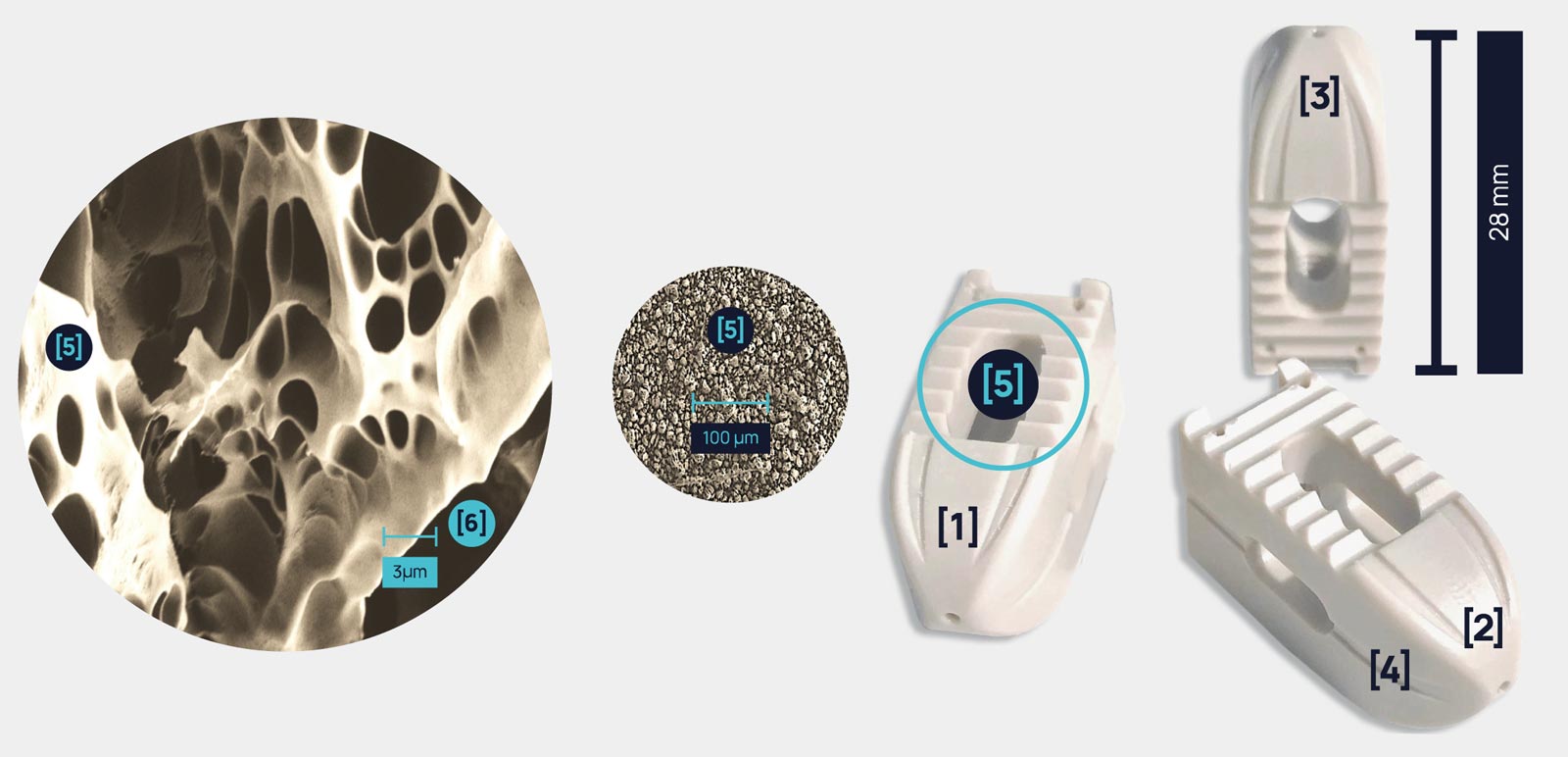
spineFuse implants follow the patient’s anatomy of the spine and are designed to restore the natural shape of the spinal column. Sliding struts [1] on the implants body and it’s bullet nose [2] guarantee an easy insertion. The anatomical implant design [3] guarantees a perfect fit. Carved out bone-anchorage struts [4] allow bone cells to better adhere to the implant’s surface by providing ideal grip. MBT surface modification and finish [5] provide for an enlarged surface topography [6] resulting in optimal primary stability and best anchorage of the implant – avoiding migration and subsidence.
stimOS spineFUSE MBT POROUS
Giving implants an open 3D porous surface
[01] SLIDING STRUTS
[02] BULLET NOSE
[03] ANATOMICAL IMPLANT DESIGN
[04] CARVED BONE ANCHORAGE STRUTS
[05] MBT OPEN POROUS SURFACE MODIFICATION: 100% SURFACE COVERAGE
[06] ENLARGED SURFACED TOPOGRAPHY
spineFuse Instruments
Sterile delivered Instrument Set
spineFuse instruments are designed to guarantee exact placement of spinFuse implants. Instrument sets can be provided sterile packed or for re-use.

Manufacturing Material Support
Additional Services from our Polymer Experts
We assist our customers in defining material specifications to choose the optimal polymer matching best the requirements for YOUR product: implant, instrument or surgical tool: The right choice of material makes the difference.
Product Qualification Support
Implementing International Quality Standards
Our regulatory specialists help YOU to qualify YOUR medical device. No matter if conventional implant, instrument or tool – or patient specific medical device, component or accessory, together we define the regulatory strategy to meet international standards and to comply with the requirements given by the Medical Device Regulation / MDR. To address patient specific implants and smart implant surfaces, stimOS has defined and set effective the quality standard S.P.E.L.
This was done in close cooperation with Notified Bodies and Certification Agencies to provide our customers best-in-class regulatory expertise.
Patient Specific Implants
stimOS Competence Center offering Best-in-Class Solutions
stimOS assists its customers in exactly defining and validating their 3D printing parameters to produce and market products qualified for the use as medical devices – serial or patient specific implants or instruments – or to be used as assisting tools in planning and executing surgery. stimOS Competence Center Services offer design and development work, printing services (together with leading industrial partners), surface modifications and certification services.